HPV testing
High-risk subtypes of human papillomavirus (HR-HPV) are linked to the development of abnormal cells and may cause cervical cancer. In 2017, the UKNSC recommended that high-risk Human papillomavirus (HR-HPV) testing should be the first (primary screening test).
Compared to cytology, HR-HPV testing has been shown to reduce the risk of developing cervical cancer through increased sensitivity for underlying disease.
In 2019, the UK moved to primary testing for HR-HPV, reserving cytology for women who test HR-HPV positive (also called reflex cytology).
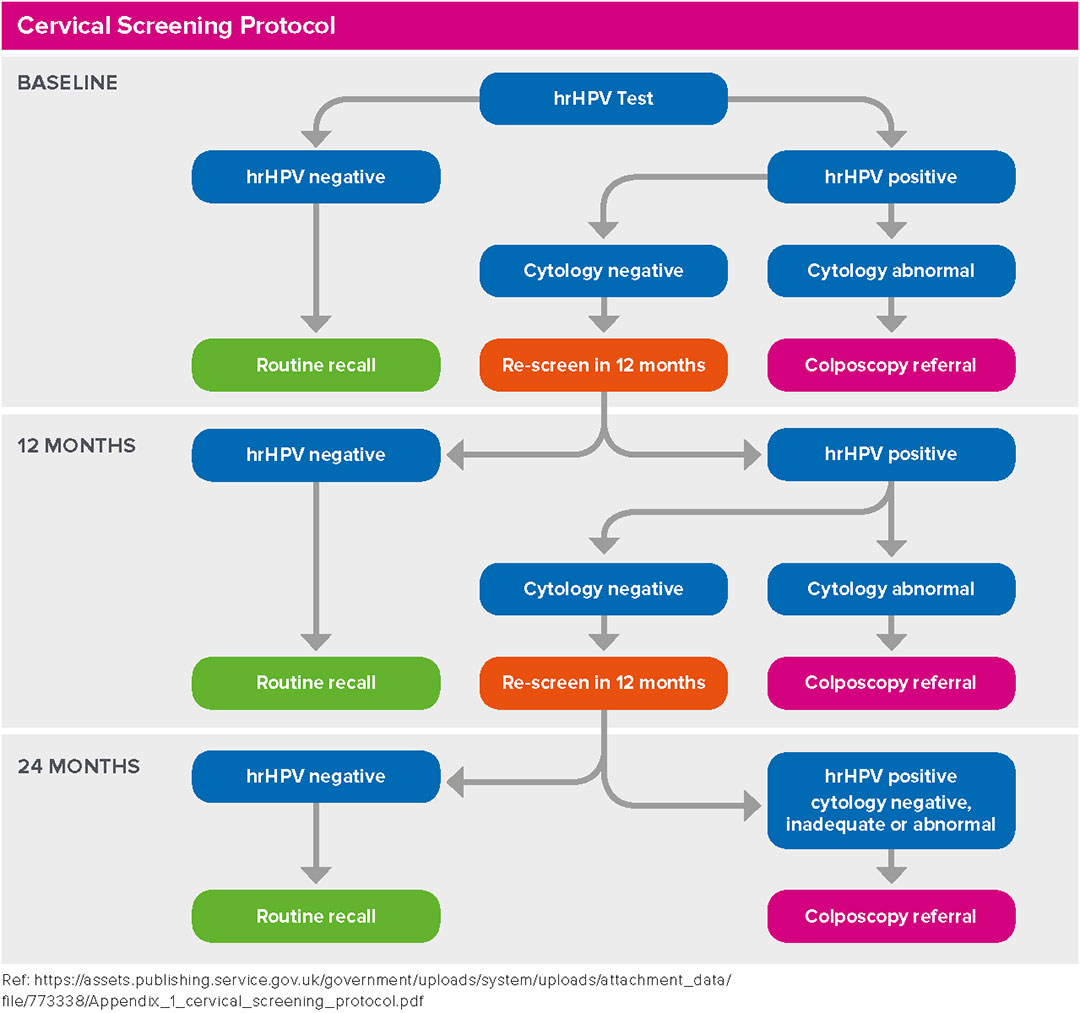
Women will be managed according to the protocol below.
- HR-HPV NOT DETECTED: no further testing is required – Return to Routine Recall.
- HR-HPV DETECTED: reflex cytology will be processed from the same ThinPrep Vial. If the cytology result from this sample is abnormal, the recommendation is to refer to colposcopy regardless of the cytology grade.
- HR-HPV DETECTED/CYTOLOGY NEGATIVE: Repeat in 12 months.
12M HR-HPV DETECTED/CYTOLOGY NEGATIVE: Repeat in 12 months.
24M HR-HPV DETECTED/CYTOLOGY NEGATIVE: Refer to colposcopy.
Cervical Screening Protocol
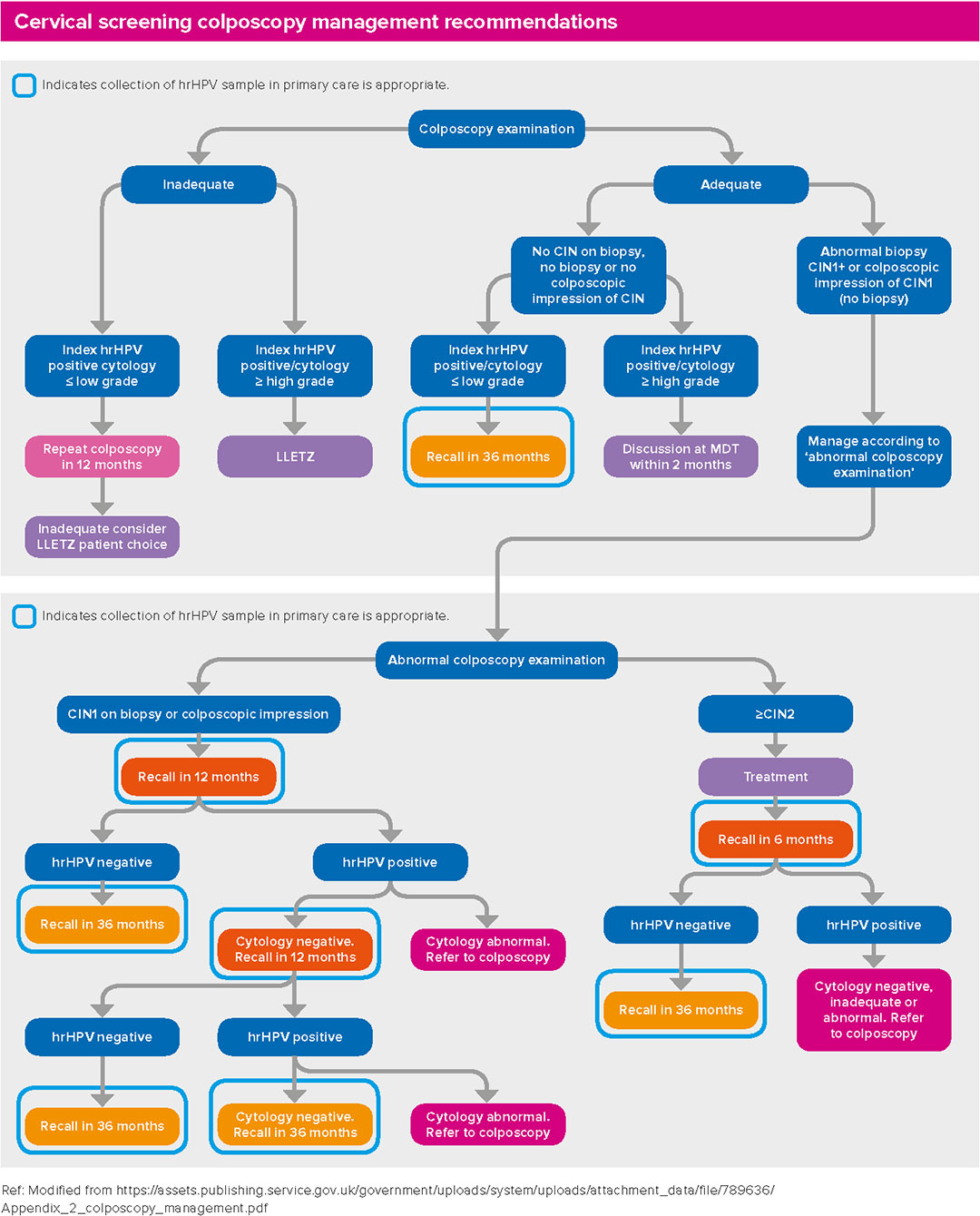
Cervical screening colposcopy management recommendations
See also: Cervical screening care pathway – GOV.UK